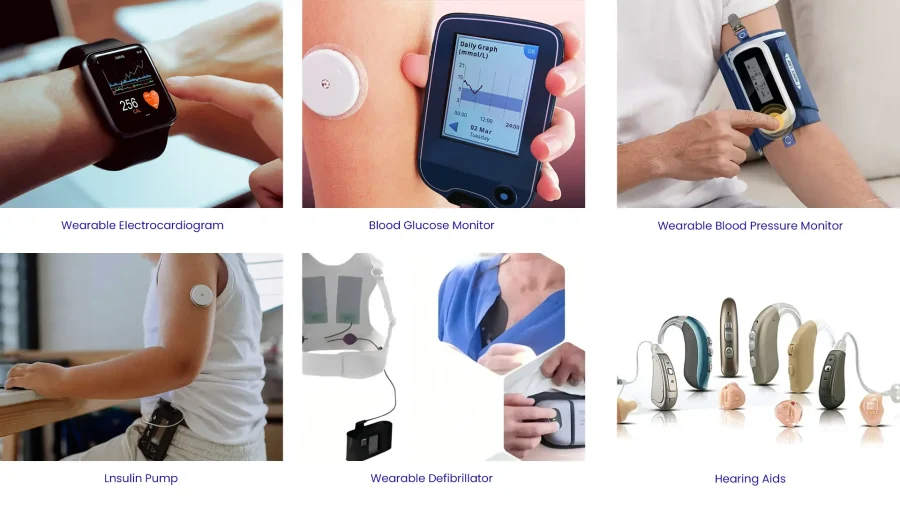
Wearable medical devices integrate with human body, capturing health data such as blood glucose, blood pressure, heart rate, blood oxygen, body temperature, and converting these signals into actionable digital insights. Rechargeable lithium-ion battery power these devices by enabling lightweight, comfortable, long-lasting, and safe operation.
However, battery energy density, compact design, mechanical flexibility, and medical-grade safety still limit the size, data accuracy, and reliability of wearable medical devices. Once connected to medical IoT(IoMT), batteries must deliver stable power, support high pulse loads from multiple sensors and wireless communication, and ensure long-term reliable operation. These requirements are critical for large-scale manufacturing and deployment.
This article will analyze medical wearable device battery design challenges, propose feasible engineering solutions, and share the latest trends in medical IoT and next-generation battery technologies.
How Medical IoT(MIoT) Market Drives the Demand for Medical Wearable Device Battery Pack
Alongside the rapid growth of the Medical Internet of Things (MIoT) market, the demand for medical wearable devices has surged. These devices not only need to accurately collect and transmit users’ health data but also must ensure stable operation to support remote patient monitoring, chronic disease management, and daily health tracking.
This market trend drives high demand for battery performance, which includes compact size, extended runtime, stable voltage output, and reliable safety. These features are all critical for medical wearable device battery design.
The table below summarizes the recent market demand trends for lithium-ion batteries in medical wearable devices, along with the key priorities for customers when selecting a battery.
| Market Demand Trend | Why it Matters for Li-ion Battery Manufacturers | Customer Priorities |
| Growth of wearable medical devices | Increasing demand for compact, reliable batteries for continuous monitoring devices | Size, comfort, long runtime, safety compliance |
| Adoption of multi-parameter sensors (heart rate, SpO₂, temperature, glucose) | Devices require stable power for multiple sensors over extended periods | Battery life, low self-discharge, consistent voltage |
| Continuous skin-contact usage | Devices need safe, lightweight, and ergonomic batteries | Thermal safety, lightweight, flexible form factor |
| Expansion of remote patient monitoring & home care | Devices operate outside clinical settings, requiring dependable energy supply | Long-term reliability, robustness under daily activities |
| Rising chronic disease management and elderly care market | Specialized applications drive need for custom battery pack | Custom capacity, form factor, regulatory compliance |
| Integration with IoT and wireless connectivity | Real-time data transmission increases energy consumption | Sufficient power for continuous communication, stable voltage output |
5 Main Challenges for Designing Medical Wearable Devices Battery
Wearable medical device batteries have much stricter requirements than consumer electronics. The following outlines five major challenges:
High Energy Density and Ultra-Compact Dimensions
Wearable medical device batteries need to strike a balance between compact size and high energy density, which ensures long battery life, comfortable wear.
Key challenges include:
- Long runtime needs: medical-grade tracking that goes on all the time, like in ambulatory blood pressure and glucose monitors, needs a lot of energy. Any delay in power can put patients at risk.
- Extremely restricted space: The battery, BMS, sensors, and circuitry must all fit into the small device volume. Without giving up safety features or volume, the battery has to stay small and light.
High Pulse Loads in Medical IoT Wearable Battery
Because it powers many sensors and Bluetooth, Wi-Fi, NFC, and ZigBee modules for wireless transmission, the battery has to be able to handle the following power demands:
- High peak power loads: When you run several functions at once, the current spikes often, which can make the system less stable.
- Accelerated aging: Repeated pulsed loads shorten cycle life and hasten battery breakdown.
- Voltage instability: Rapid voltage drops might result in faulty sensor readings and data output.
Humidity, Sweat Corrosion, and Heat Dissipation Challenges
Wearable devices expose batteries to sweat, moisture, high temperatures, and limited airflow.
Key challenges include:
- Sweat-induced corrosion that increases internal impedance
- Restricted heat dissipation due to tight contact with the skin
- Faster capacity degradation and more severe cycle life reduction
- More frequent activation of PTC/BMS safety protections
Rigid Electrodes and Skin-Conforming Flexible Electrodes
Wearable medical devices, like patch-type vital signs monitors, require batteries that fit closely to the body for comfort and reliable sensor performance. However, traditional lithium-ion batteries(pouch, cylindrical, and coin cells) with rigid electrodes often fail under bending or stretching, leading to electrode delamination and capacity loss.
Recent research shows that even ultrathin Li-ion pouch cells experience severe degradation from repeated flexing, proving that rigid batteries are prone to failure in wearable applications (Kim & Chan, 2024).
Flexible pouch battery designs help solve this, but they often sacrifice energy density and cycle life. Choosing the right battery for wearable devices requires balancing flexibility, durability, and sufficient power for continuous monitoring.
Safety Requirements for Medical Wearable Batteries
Wearable medical devices require continuous operation and therefore rely heavily on stable battery performance. These devices require strong electrical, thermal, and mechanical safety because they are subjected to long periods of movement, high temperatures, and ordinary wear and tear.
Key Points for Battery Safety in Wearable Medical Devices
Thermal Management
- Temperature Monitoring: Integrated NTC sensors and software algorithms enable early detection of abnormal conditions.
- Active/Passive Thermal Management: Graphite sheets, phase change materials (PCM), and heat spreaders help manage heat within confined spaces.
- Mechanical Protection: Reinforced enclosures and flexible PCBs prevent puncture damage and material fatigue.
Certifications for Medical Wearable Batteries
Wearable batteries require an ultra-thin form factor, robust safety features, and stable power output. Compliance with global standards—IEC 60601, IEC 62133, UN 38.3, and UL 2054—is essential for market entry.
| Certification | Test Focus | Description |
| IEC 60601-1 | Medical electrical system safety, leakage current, mechanical hazards, single-fault safety | Pack must integrate safely with the medical device system and remain safe under single-fault conditions. |
| IEC 62133-2 | Rechargeable Li-ion cell & battery pack safety | Prevent leakage, fire, and venting under user misuse and mechanical stress |
| UN 38.3 | Transport safety for lithium batteries | Ensure safe worldwide shipping for wearable devices. |
| UL1642 | Cell-level abuse tests: crush, short, impact, forced discharge | Needed by many North American medical customers for reliable lithium cells. |
| UL 2054 | Pack-level safety: insulation, enclosure strength, dielectric withstand, abnormal charging | Preferred safety standard for battery packs in North America. |
| IEC 60601-1 Environmental requirements/IEC 60068-2 | Humidity, thermal cycling, sweat exposure simulation | Sweat corrosion increases impedance and accelerates capacity fade. |
| IEC 60068-2 (shock, vibration, drop, compression) | Mechanical robustness: shock, vibration, drop, compression | Prevent internal weld cracking and electrode deformation in daily wear conditions. |
The article presents complete guide to medical device battery certifications to see how our custom battery packs meet battery medical global standards.
Battery Technology Strategies for Medical Wearable Devices
Advanced Lithium-ion Battery Chemistry Materials
The appropriate lithium-ion battery chemistry material for medical wearable devices is a critical solution for enhancing overall device performance. Choose right chemistry based on your current application demands.
| Battery Chemistry | Key Features | Advantages for Wearable Medical Devices | Potential Challenges |
| Lithium Manganese Coin Cell(CR1216, CR1025, CR920) | Compact size, low self-discharge, good high-temperature stability, low leakage risk, stable voltage | Suitable for space-constrained devices requiring high data accuracy, such as patch-type monitors and micro-sensors | Low capacity (20-35 mAh), typically disposable |
| Nickel Metal Hydride (NiMH) | High temperature resistance, long cycle life, high single-cell capacity, low internal resistance, supports fast charging (1C-2C) | Ideal for high-end medical devices; cycle life can exceed 1000 cycles; suitable for scenarios requiring rapid charging, such as emergency equipment | Relatively high self-discharge rate |
| NMC (LiNiMnCoO2) | High energy density, rechargeable | Supports high-power peak loads, long runtime. Suitable for multi-sensor connected devices | Higher Cost. Thermal management require BMS for safety |
| LiFePO4 (LFP) | Moderate energy density and excellent thermal stability | High safety, long cycle life, stable voltage under dynamic load | Heavy design, low energy density and limited runtime for high-power devices |
| LiPo (Lithium Polymer) | Flexible form factor, rechargeable | Lightweight design supports module configurations and integrates into wearable device form factors | LiPo batteries have higher self-discharge than LiFePO4, require careful handling to avoid punctures |
Intelligent Battery Management System
Medical wearable devices increasingly require a full Battery Management System (BMS) rather than a simple Protection Circuit Module (PCM).
- Accurate state-of-charge (SoC) and state-of-health (SoH) monitoring
- Integrated temperature monitoring
- Over-current and short-circuit protection
For a deeper technical explanation, explore our in-depth guide to Battery Management Systems.
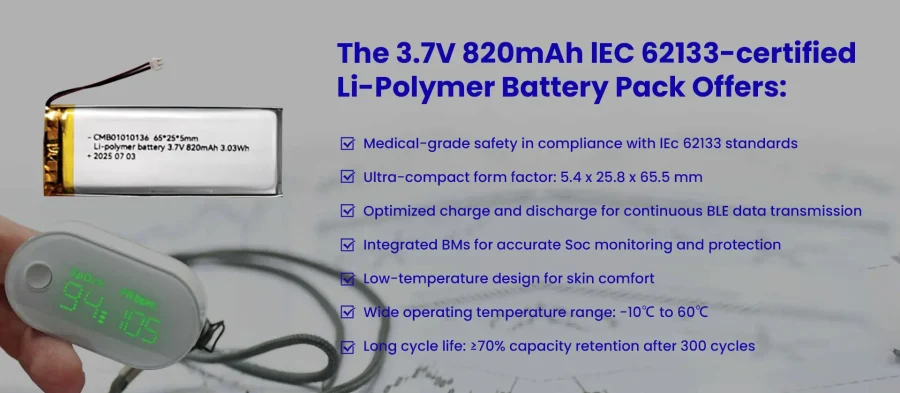
Case Study: 3.7V 820mAh IEC 62133 Certified LiPO Battery for Wearable Medical Devices
A wearable device manufacturer requires a compact and safe battery for its multi-parameter IoMT patch, which monitors heart rate, body temperature, blood oxygen (SpO₂), respiratory rate, and activity levels. The battery must support 5–7 days of continuous operation, enable Bluetooth Low Energy (BLE) data transmission, and maintain a low-temperature, ultra-thin form factor for patient comfort.
Customer Requirement
A multi-parameter IoMT patch continuously monitors vital signs such as heart rate and activity, requiring long-lasting and safe power to support uninterrupted operation. The following questions are our customer concerns:
- Can you offer ultra-compact battery that fits into limited medical wearable space?
- Is the battery already compliant with major global safety certifications for medical devices?
- Can it support the charging and discharging performance required for continuous monitoring?
- Will the battery pack stay stable across typical wearable temperature conditions?
- Does it offer reliable cycle life for long-term medical use?
CMB Solution & Key Design Highlights
The 3.7V 820mAh IEC 62133-certified Li-Polymer battery pack offers:
CM Batteries: Your Reliable Wearable Medical Device Battery Engineering Partner
CM Batteries provides custom lithium battery solutions for wearable medical devices. With strong engineering expertise and advanced Battery Management System (BMS) technology, we design lithium-ion battery packs that are safe, compact, and highly reliable, tailored to each device’s size, power requirements, and certification needs. If you have any requests, please contact us.