Lithium-ion batteries have indeed become an essential component of modern life, quietly powering the devices and tools we rely on daily. In a world without reliable battery technology harmper many advancements in mobility, connectivity, and emergency response .
A battery is, in its simplest form, a device that stores energy chemically, converting it into electrical energy. It can be as basic as a single electrochemical cell or as complex as a series of cells working in tandem to deliver higher voltages or larger capacities.
A Brief History of Rechargeable Lithium-Ion Batteries
Rechargeable lithium-ion battery chemistries have evolved, with four major types that each brought distinct advances and enabled new applications: Lead Acid, Nickel Cadmium (Ni-Cd), Nickel Metal Hydride (Ni-MH), and Lithium Ion. Let’s look at a brief history of each.
- Lead Acid Batteries

Lead-acid batteries, formally introduced in the 1800s, have become one of the most widely used and recognized battery chemistries worldwide. Due to their low production costs, affordable raw materials, strong performance, and long cycle life, they currently represent around 40-45% of the global battery market. Using Lead-acid batteries in start automobiles, trucks, motorbikes, and ATVs are normal.
- NiCad Batteries

Nickel-cadmium (Ni-Cd) batteries, created and patented by Waldemar Jungner in 1899, have a long history as a reliable, well-researched rechargeable battery technology. Their unique properties—such as low internal resistance and the ability to deliver high surge currents—make them valuable in several consumer, commercial, and industrial applications, because of their relatively low internal resistance and ability to provide extremely high surge discharge currents. Because nickel and cadmium are classified as hazardous materials, disposing of Ni-Cd batteries responsibly. Many countries have regulations requiring the recycling of Ni-Cd batteries to prevent cadmium from contaminating landfills. Consumers and businesses should dispose of Ni-Cd batteries at designated recycling centers.
- NiMH Batteries

Nickel-metal hydride (NiMH) batteries appeared in the early 1990s and developed as a more efficient and environmentally friendly alternative to nickel-cadmium (Ni-Cd) batteries. With a cell voltage of 1.2 VDC, identical to that of Ni-Cd, NiMH batteries can directly replace Ni-Cd batteries in many applications without any modifications to existing devices. This compatibility, coupled with their higher energy density and reduced environmental impact, has allowed NiMH batteries to dominate in consumer electronics, largely overtaking Ni-Cd batteries.
- Lithium Ion Batteries

Lithium-ion (Li-Ion) batteries have become the dominant technology in portable electronics, powering everything from smartphones and laptops to smartwatches and vape pens. Emerging in the mid-1990s, Li-Ion batteries quickly overtook nickel-metal hydride (NiMH) batteries as the primary choice for mobile devices due to their superior energy density, lightweight structure, and rechargeability.
The term “Lithium-Ion battery” actually encompasses a variety of chemistries, each with unique properties tailored to different applications. Drive these differences by the choice of positive and negative electrode materials and how lithium ions transfer through the non-aqueous electrolyte. This range allows for the customization of attributes like energy density, safety, and charging speed.
How to define a lithium-ion battery?
Lithium-ion (Li-Ion) batteries have become the go-to choice for consumer electronics due to their high energy densities, moderate to high cell voltages, and lightweight properties compared to older battery chemistries like lead-acid, nickel-cadmium (Ni-Cd), and nickel-metal hydride (NiMH). This technology also powers modern innovations like the LED bathroom mirror.
Li-Ion batteries represent a transformative technology that is still early in its lifecycle but already widely adopted. As costs decline and performance improves, Li-Ion expands its role across consumer electronics, EVs, and large-scale energy storage. Given the ongoing innovation in Li-Ion chemistry and design, these batteries have the potential to meet future demands across various industries while supporting a transition to more sustainable energy solutions. As a leading custom battery pack manufacturer, CMB is proud to be a pioneer of the power solutions of the future.
Non-rechargeable Primary Lithium Batteries
Primary lithium batteries have been in use since the 1970s, primarily serving portable power needs in industrial, military, and consumer applications. They stand out for their long shelf life, low maintenance, and high energy density. Some primary lithium batteries can retain capacity for up to 10–20 years, making them a reliable backup power source. These batteries can also withstand storage at elevated temperatures, with some types tolerating up to 70°C, which is beneficial in environments subject to extreme conditions.
The most popular basic lithium battery chemistries in the market are lithium desulphated (LiFeS2) and lithium manganese dioxide (LiMnO2).
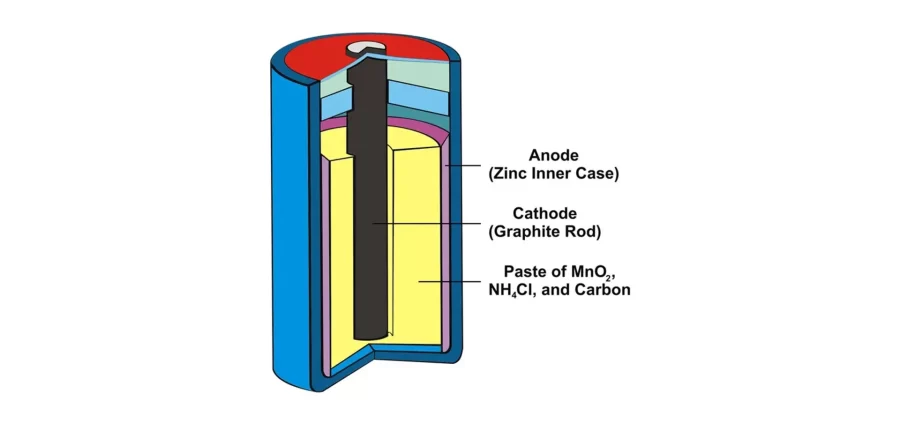
Lithium-ion Battery Types
Rechargeable lithium batteries fall into two main categories: lithium metal batteries and lithium-ion (Li-Ion) batteries.
Lithium reacts with the electrolyte in a lithium metal battery, causing dendrites to develop on the electrode’s surface.
The second form of rechargeable lithium battery is known as a lithium-ion battery. The negative terminal of a Li-Ion battery is made of a carbon-based substance such as graphite. The terminal can be made of any type of alloy or substance as long as it allows for interconnectivity through lithium storage in its structure.
Rechargeable Lithium-Ion Batteries FAQs
Q: Why do they use lithium in their batteries?
A: Lithium is widely used in batteries due to its unique properties that contribute to efficient, high-performing battery systems
Q: What is the shelf life of a Lithium-Ion battery in storage?
A: Lithium-ion batteries typically have a shelf life of 3 to 6 years in storage, depending on storage conditions and the battery’s specific chemistry. Self-discharge, which leads to capacity loss over time, occurs at a rate of 2% to 5% per month.
Q: Are all lithium-ion cells rechargeable?
A: Not all lithium-based cells are rechargeable. Lithium-ion batteries are indeed secondary cells, which means they are rechargeable. However, there are also lithium primary cells, using for single-use only once. Attempting to recharge primary lithium cells is extremely dangerous, as it can lead to overheating, swelling, and even explosion. If there is any doubt about whether a lithium cell is rechargeable, it’s crucial to check the specifications provided by the manufacturer.
Q: What is the distinction between Li-Ion and LiPo/Lithium Polymer?
A: Li-Ion is ideal for applications prioritizing energy density and cycle life, while LiPo is often the choice for applications needing flexibility in shape, safer discharge at high rates, and resilience in high-performance environments.










2 thoughts